Smart Solutions for the Life Science & Regulatory Industry
Optimizing efficiency and compliance with advanced technologies
Utthunga empowers life sciences and regulatory organizations with future-ready solutions that unite engineering expertise, intelligent automation, and digital capabilities. Our approach balances technological advancement with strict adherence to regulatory standards, reducing complexity, strengthening product quality, and delivering consistent operational excellence.
Building on this foundation, and in step with industry-leading trends such as AI-driven regulatory automation, digital process simulation, and cloud-enabled data integrity, we deliver measurable gains in accelerating drug development and enhancing quality control processes. The outcome: faster time-to-market and assured compliance across global standards.
Our Core Competencies
 Conduct thorough audits aligned with GxP, FDA, EMA, and ICH guidelines to identify non-conformances, improve documentation practices, and ensure inspection readiness across labs and manufacturing facilities.
Conduct thorough audits aligned with GxP, FDA, EMA, and ICH guidelines to identify non-conformances, improve documentation practices, and ensure inspection readiness across labs and manufacturing facilities.
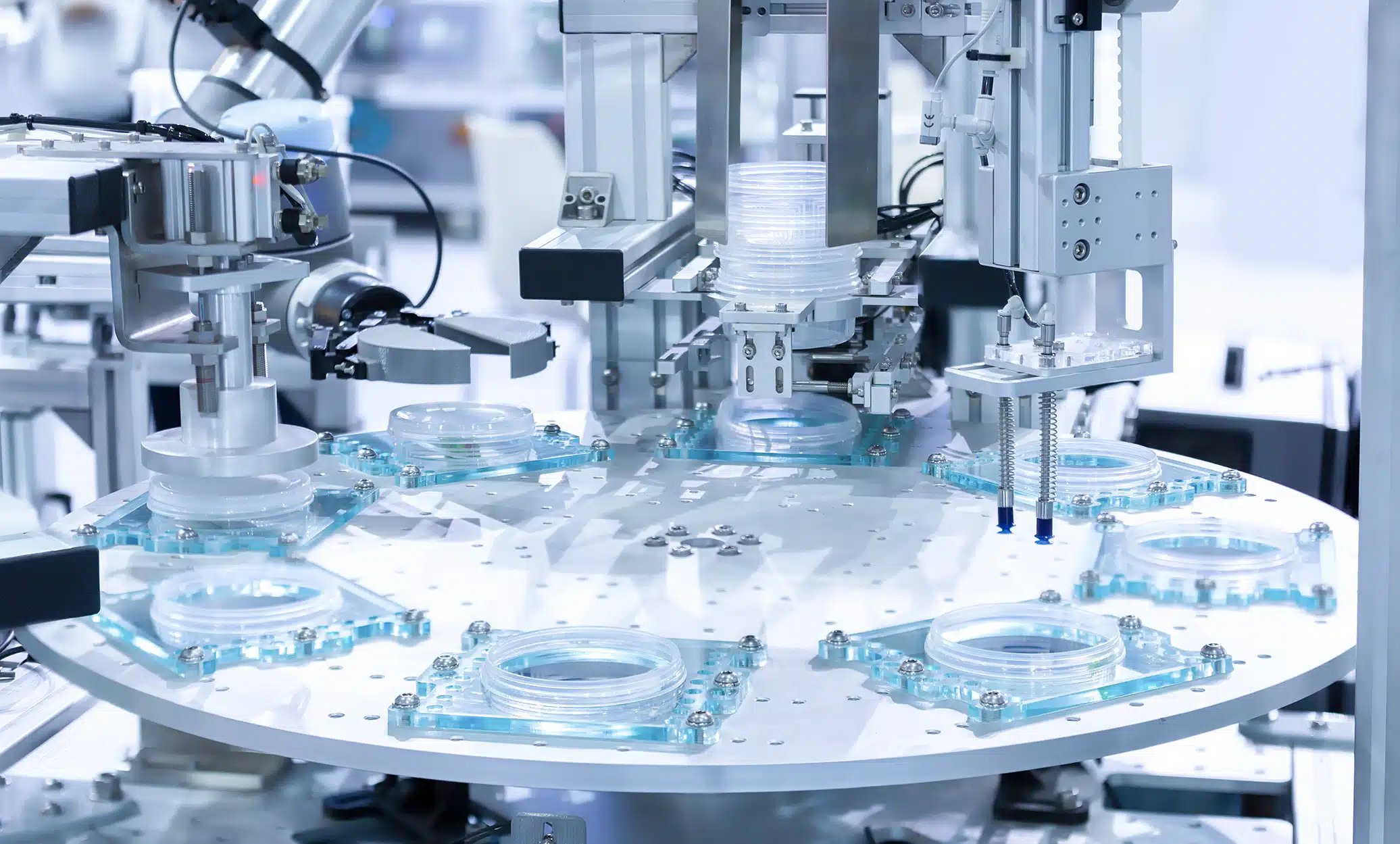 Implement SCADA, MES, and LIMS-driven automation for cleanrooms, bioreactors, and analytical labs to enhance throughput, ensure data integrity, and maintain compliance with 21 CFR Part 11.
Implement SCADA, MES, and LIMS-driven automation for cleanrooms, bioreactors, and analytical labs to enhance throughput, ensure data integrity, and maintain compliance with 21 CFR Part 11.
 Enable digital transformation with electronic batch records, cloud-based validation, and end-to-end traceability, ensuring ALCOA+ principles and compliance with global regulatory frameworks like EU Annex 11 and PIC/S.
Enable digital transformation with electronic batch records, cloud-based validation, and end-to-end traceability, ensuring ALCOA+ principles and compliance with global regulatory frameworks like EU Annex 11 and PIC/S.
 Provide expert consultation across regulatory submissions, market authorization, QMS setup, and lifecycle management—supporting compliance with FDA, MHRA, WHO, and CDSCO requirements for drugs and medical devices.
Provide expert consultation across regulatory submissions, market authorization, QMS setup, and lifecycle management—supporting compliance with FDA, MHRA, WHO, and CDSCO requirements for drugs and medical devices.
 Design, optimize, and qualify GMP-compliant production lines and HVAC systems for sterile manufacturing, leveraging cleanroom classification, process validation, and contamination control principles.
Design, optimize, and qualify GMP-compliant production lines and HVAC systems for sterile manufacturing, leveraging cleanroom classification, process validation, and contamination control principles.
 Deliver turnkey EPCC projects for laboratories and manufacturing plants—covering design, procurement, clean utility systems, and commissioning—ensuring cGMP alignment, validation, and audit-readiness from day one.
Deliver turnkey EPCC projects for laboratories and manufacturing plants—covering design, procurement, clean utility systems, and commissioning—ensuring cGMP alignment, validation, and audit-readiness from day one.
 Develop dynamic simulation models for aseptic processing, HVAC zoning, and deviation scenarios to support equipment validation, operator training, and performance optimization in GxP-regulated environments.
Develop dynamic simulation models for aseptic processing, HVAC zoning, and deviation scenarios to support equipment validation, operator training, and performance optimization in GxP-regulated environments.
Lab & Manufacturing Automation Solutions
Implement SCADA, MES, and LIMS-driven automation for cleanrooms, bioreactors, and analytical labs to enhance throughput, ensure data integrity, and maintain compliance with 21 CFR Part 11.


Digitalization for Data Integrity & Traceability
Enable digital transformation with electronic batch records, cloud-based validation, and end-to-end traceability, ensuring ALCOA+ principles and compliance with global regulatory frameworks like EU Annex 11 and PIC/S.
Specialized Regulatory Consultation Services
Provide expert consultation across regulatory submissions, market authorization, QMS setup, and lifecycle management—supporting compliance with FDA, MHRA, WHO, and CDSCO requirements for drugs and medical devices.


Process Engineering for GMP Environments
Design, optimize, and qualify GMP-compliant production lines and HVAC systems for sterile manufacturing, leveraging cleanroom classification, process validation, and contamination control principles.

Simulation for Validation & Operator Training
Develop dynamic simulation models for aseptic processing, HVAC zoning, and deviation scenarios to support equipment validation, operator training, and performance optimization in GxP-regulated environments.
Our Smart Solutions for the Life Science & Regulatory Industry
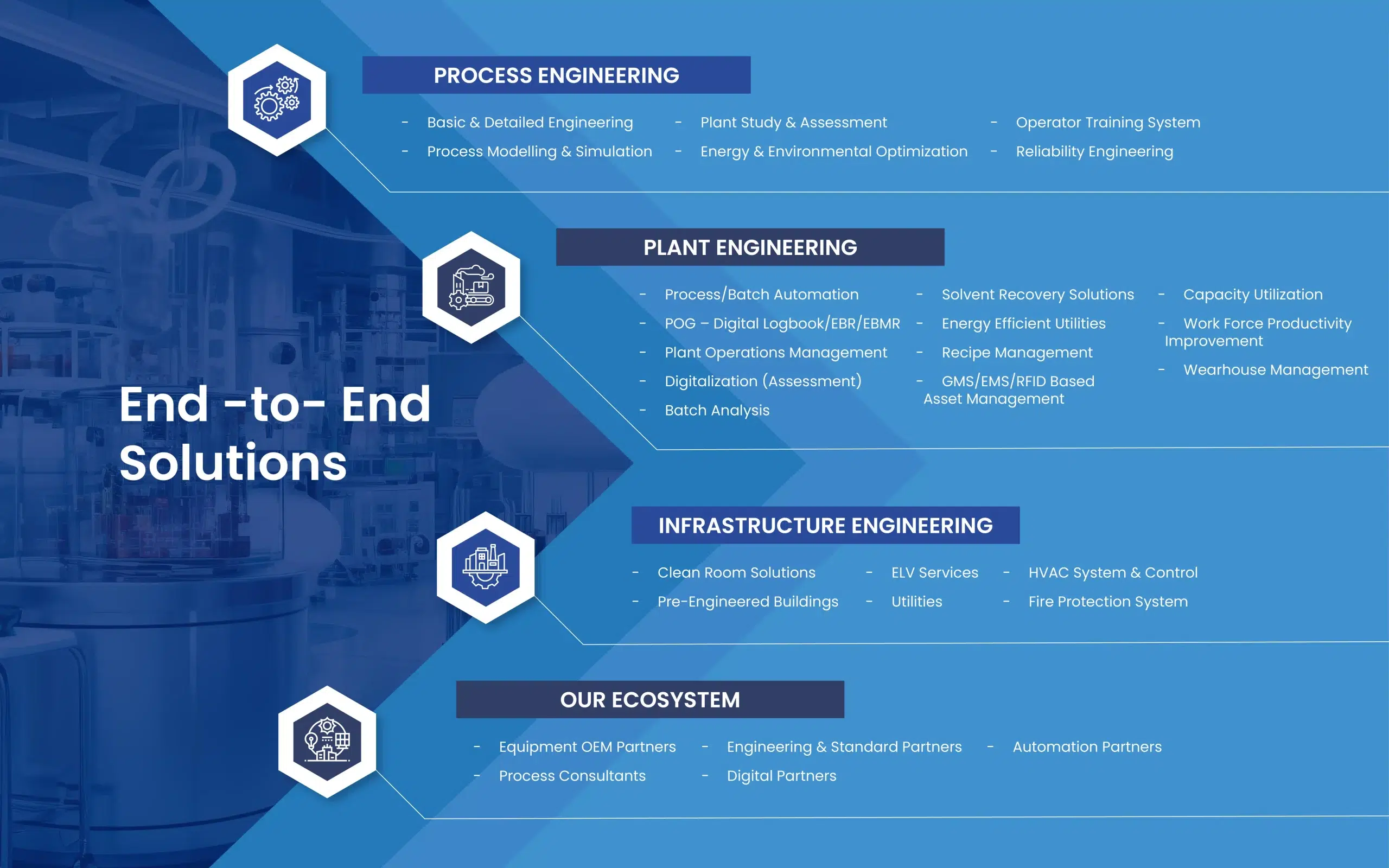
Benefits of Our Smart Solutions for the Life Science & Regulatory Industry

Accelerated Drug Development & Market Access
Our automation-driven platforms streamline clinical trial execution, regulatory submissions, and eCTD workflows—accelerating time-to-market for pharmaceuticals, biologics, and advanced therapies while maintaining full regulatory alignment.
Compliance Excellence Across GxP Environments
We enable consistent compliance with global regulatory standards like FDA, EMA, ICH, and WHO through digital validation, audit readiness, and robust cGxP-aligned documentation and reporting frameworks.
Optimized GMP Operations & Quality Control
Our integrated solutions enhance batch execution, deviation management, and real-time monitoring—minimizing manual errors and enabling scalable, efficient GMP operations across manufacturing, labs, and quality systems.
